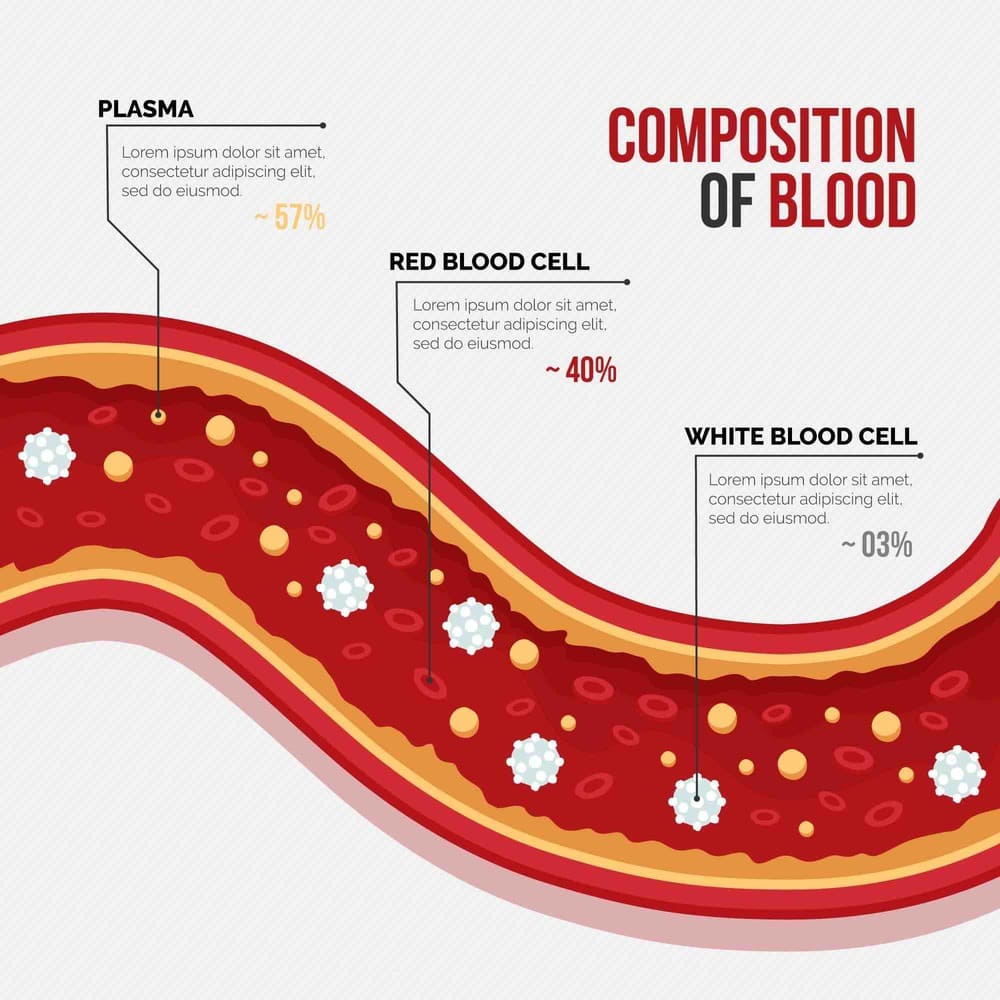
Inflammation is the body’s natural immune response to infection, injury, toxins or other harmful stimuli. In a healthy (“physiologic”) setting, inflammation is essential for healing and repair; it mobilizes immune cells and molecular mediators to eliminate pathogens and debris. For example, fever during an infection is a sign that the body’s inflammatory system is working properly. However, when inflammation becomes excessive or chronic, it turns pathological, contributing to tissue damage and disease. The classical signs of inflammation – redness (rubor), heat (calor), swelling (tumor), pain (dolor), and loss of function (functio laesa) – reflect increased blood flow, vascular permeability, and chemical signals in affected tissues. In summary, inflammation is the body’s innate defense mechanism: a coordinated cascade of vascular and cellular events aimed at restoring tissue homeostasis, but which must be tightly controlled to avoid collateral damage.
Types and Mechanisms of Inflammation
There are two major forms of inflammation – acute and chronic – distinguished by their duration and cellular mechanisms. Acute inflammation is the immediate, short-term response (lasting hours to days) that follows tissue injury or infection. It is mediated by the innate immune system and involves rapid vasodilation, increased capillary permeability, and recruitment of neutrophils to the site of injury. Early chemical mediators include histamine, nitric oxide and prostaglandins, which cause blood vessels to dilate (leading to redness and warmth) and become more permeable (allowing fluid and immune cells to enter the tissue). Phagocytic leukocytes (primarily neutrophils and macrophages) then engulf pathogens or debris, aided by plasma proteins (complement, kinins, acute-phase reactants). In acute inflammation, resolution and tissue repair usually occur within days: dead cells are cleared and healing commences.
By contrast, chronic inflammation is a sustained, long-term process (lasting weeks, months or years) that arises if an acute response fails to eliminate the offending agent, or if low-grade stimuli persist. Chronic inflammation is characterized by infiltration with mononuclear cells (macrophages, lymphocytes, plasma cells), ongoing cytokine production, and gradual tissue remodeling or fibrosis. Over time, these cells release growth factors and enzymes that can damage normal tissue architecture. For example, in rheumatoid arthritis, chronic synovial inflammation leads to joint erosion; in atherosclerosis, persistent arterial wall inflammation promotes plaque formation. While acute inflammation serves a protective physiological role in healing, chronic inflammation is essentially pathological, contributing to disease progression.
Figure: Acute inflammation (illustrated on the left) is an immediate, short-lived response to tissue injury, whereas chronic inflammation (right) persists and underlies many chronic diseases.
Biological Mechanisms
At a molecular level, inflammation involves a complex interplay of cells, mediators, and signals. The process begins when injury or infection triggers cells (e.g. macrophages, dendritic cells, mast cells) to release pro-inflammatory cytokines (such as TNF-α, IL-1β, IL-6) and chemokines. These mediators induce vasodilation (via nitric oxide and histamine) and increase vascular permeability (via bradykinin, leukotrienes), causing the classic signs of heat and swelling. Chemical mediators also stimulate pain receptors (sensitizing nociceptors) and promote expression of adhesion molecules on vascular endothelium. These molecules (selectins, integrins) facilitate leukocyte extravasation: circulating neutrophils and monocytes stick to vessel walls and migrate into the tissue to phagocytose debris and pathogens.
Simultaneously, the acute phase response is activated in the liver, producing proteins like C-reactive protein (CRP), fibrinogen, and complement components. CRP, for instance, increases dramatically during acute inflammation and serves as a clinical marker of systemic inflammation. As the response progresses, neutrophils predominate in the first 24–48 hours, followed by monocytes that differentiate into tissue macrophages. If the inciting insult is cleared, anti-inflammatory signals (e.g. IL-10, TGF-β) promote resolution: neutrophils undergo apoptosis, macrophages clear debris, and tissue repair factors (fibroblasts, endothelial growth factors) regenerate normal structure.
In chronic inflammation, by contrast, the continued presence of antigen or a dysregulated immune response leads to a different set of players. Macrophages remain activated and secrete further cytokines and enzymes (e.g. metalloproteinases) that recruit lymphocytes (T and B cells) and stimulate fibrosis. Cytokines such as interferon-γ (from Th1 cells) perpetuate macrophage activation. Over months, this can lead to granuloma formation in some conditions (clusters of epithelioid macrophages surrounded by lymphocytes, as in tuberculosis or sarcoidosis). Persistent inflammation also induces angiogenesis (new vessel formation) and connective tissue deposition, which can cause scarring. In essence, unresolved inflammation “burns” tissue and sets the stage for chronic disease.
Common Causes and Risk Factors
Inflammation can be triggered by a wide range of stimuli. Acute inflammation typically follows infections (bacterial, viral, fungal, parasitic) or physical injury (cuts, burns, fractures). Classic examples include an acute bacterial appendicitis, cellulitis, or a sprained ankle. In these cases, microbial products (e.g. lipopolysaccharide) or tissue damage molecules activate innate sensors (pattern recognition receptors) and elicit a rapid response.
Chronic inflammation often arises from prolonged exposure to irritants or from immune dysregulation. Risk factors and causes include:
Persistent infections or foreign bodies: Certain infections (e.g. Mycobacterium tuberculosis, hepatitis viruses) tend to incite long-lasting inflammation. Foreign materials (silica dust, sutures, or prosthetic devices) can also chronically irritate tissues.
Autoimmune diseases: In conditions like rheumatoid arthritis, systemic lupus erythematosus or inflammatory bowel disease, the immune system inappropriately targets the body’s own cells, driving continual inflammation.
Environmental/lifestyle factors: Sedentary lifestyle, chronic psychological stress, smoking, poor diet, obesity, and disrupted sleep have all been linked to low-grade chronic inflammation. For instance, adipose tissue (especially visceral fat) secretes pro-inflammatory adipokines, explaining why obesity is a major risk factor. Diets high in trans fats, refined carbohydrates or sugar promote cytokine production, whereas diets rich in fruits, vegetables and omega-3 fats are anti-inflammatory.
Age and hormonal factors: Inflammaging refers to the gradual increase in systemic inflammatory markers with aging. Sex hormones like estrogen and testosterone normally help suppress inflammation, so hormonal decline (e.g. menopause) can slightly elevate inflammatory tone.
In summary, chronic inflammation is often driven by modifiable risk factors. Smoking, obesity, poor sleep and chronic stress rank among the top contributors. Interventions targeting these factors (weight loss, exercise, smoking cessation, stress reduction) can therefore reduce one’s inflammatory burden and disease risk.
Signs and Symptoms
The clinical presentation of inflammation depends on whether it is acute or chronic, and on the tissues involved. The five classical signs of acute inflammation – redness, heat, swelling, pain, and loss of function – are often remembered by their Latin names rubor, calor, tumor, dolor, and functio laesa. These manifest locally at the site of injury. For example:
Redness and warmth: Caused by arteriolar vasodilation, increasing blood flow to the area.
Swelling (edema): Due to plasma exudation from leaky vessels into the interstitial space.
Pain and tenderness: Result from direct nerve stimulation by bradykinin and prostaglandins, and from stretch of tissues by edema.
Loss of function: As Celsus noted long ago, severe inflammation can impair tissue function (e.g. limited joint movement).
In addition to the cardinal signs, patients may experience systemic symptoms. Acute inflammation often causes malaise and fever; chills and headache can accompany severe infections. A raised white blood cell count with a left shift (neutrophilia) is common on bloodwork. Levels of acute-phase reactants (e.g. CRP, ESR) spike, which are easily measured lab markers of inflammation.
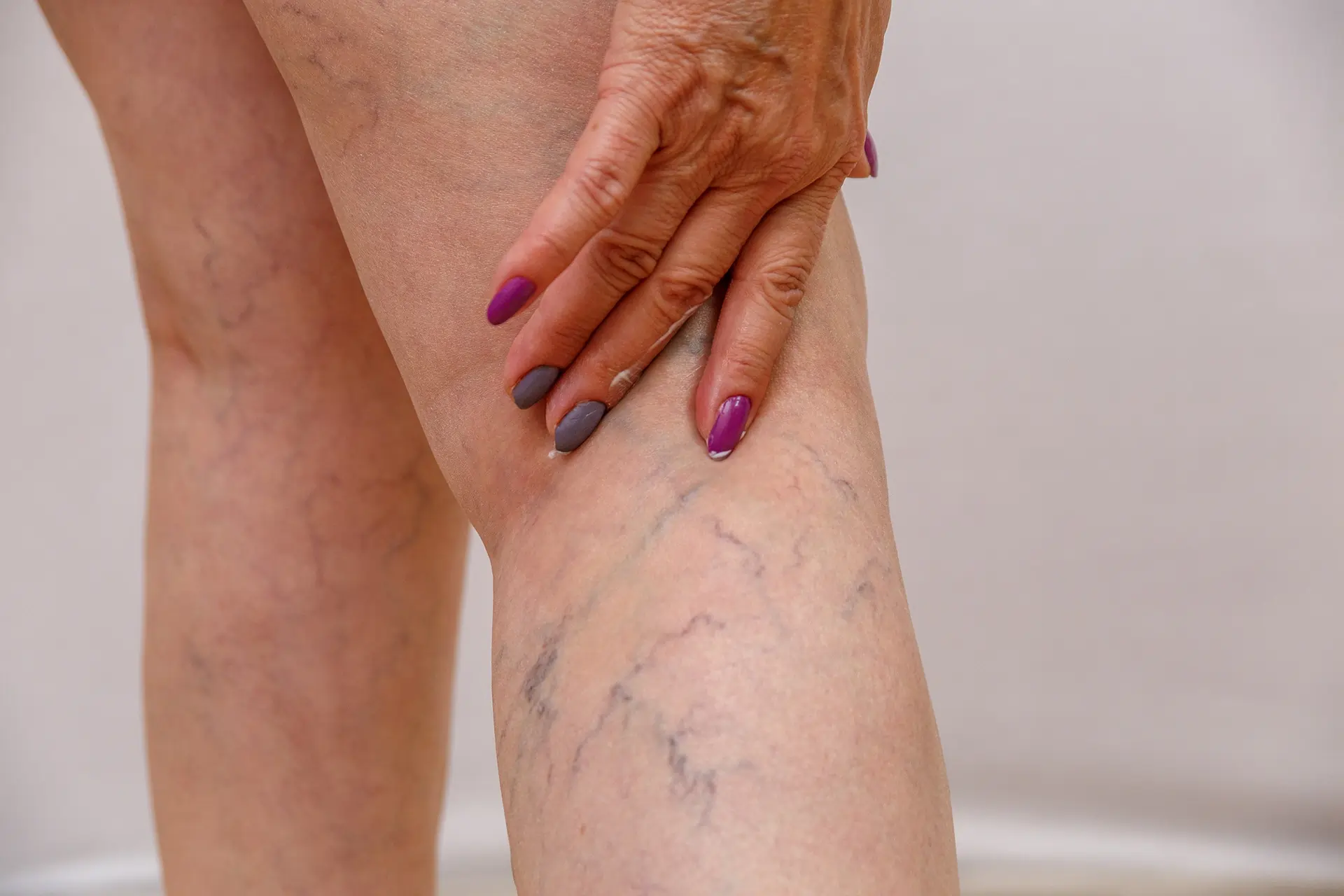
Chronic inflammation tends to cause subtler or more diffuse symptoms, sometimes making it hard to recognize. Rather than localized heat and redness, patients may have ongoing fatigue, low-grade fevers, weight loss or weight gain, and nonspecific aches. For instance, chronic gastritis or colitis may cause abdominal discomfort or diarrhea; chronic sinusitis causes congestion and headaches. In autoimmune arthritis (rheumatoid arthritis), chronic joint inflammation leads to morning stiffness and swelling. Because chronic inflammation can affect multiple organs, symptoms may include chest pain (from inflammation in the heart or pericardium), rash (e.g. lupus), mouth sores (in inflammatory bowel disease), or cognitive changes (in neuroinflammatory conditions).
In practice, redness, swelling, heat and pain at a focal site (e.g. a skin laceration or joint) usually indicate acute inflammation. More diffuse features – persistent fatigue, mild fevers, elevated inflammatory markers without clear infection – often suggest a chronic inflammatory process. Table 1 below summarizes common symptoms:
Acute inflammation symptoms: Local redness, swelling, warmth, sharp pain, limited use of the affected part.
Chronic inflammation symptoms: Fatigue, low-grade fever, vague aches, digestive issues, mood disturbances (depression/anxiety), and organ-specific symptoms depending on the disease.
Diagnostic Methods
Diagnosing inflammation involves both clinical assessment and modern testing. Initially, doctors take a careful history and perform a physical exam. They look for the classic signs locally, but also ask about systemic symptoms (fever, weight changes, etc.). Basic laboratory tests are often the first step:
Blood tests: A high white blood cell count, especially neutrophils, often accompanies acute inflammation. Serum inflammatory markers like erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are routinely measured. An elevated CRP is a sensitive indicator that inflammation is present somewhere in the body. (For example, CRP levels above 8–10 mg/L indicate significant inflammation.) These tests do not pinpoint the cause but can gauge the severity and monitor response to therapy.
Autoantibodies and serologies: If autoimmune disease is suspected, specific antibodies (e.g. rheumatoid factor, anti-CCP for rheumatoid arthritis; ANA for lupus) may be tested. Serologic tests can also identify infections (e.g. hepatitis panels, HIV) that trigger inflammation.
Tissue analysis: In some cases, a biopsy of the inflamed tissue may be needed. Pathology can show the type of inflammatory cells (acute neutrophils vs chronic lymphocytes/granulomas) and may reveal an underlying cause (e.g. vasculitis, malignancy).
For localized inflammation, imaging studies play a key role. Ultrasound can detect joint effusions or synovitis. MRI and CT scans can visualize soft tissue inflammation (e.g. in tendons or bowel). Nuclear imaging (e.g. FDG-PET) increasingly helps identify “hot spots” of active inflammation throughout the body. Endoscopic procedures (colonoscopy, bronchoscopy) may be used to directly view and biopsy inflamed mucosal surfaces.
Novel and emerging diagnostics include biomarkers of immune activation (e.g. cytokine panels) and genetic tests (e.g. HLA typing for certain inflammatory syndromes). However, CRP and ESR remain the standard for detecting systemic inflammation. Importantly, these tests must be interpreted in context – many infections, injuries, and chronic diseases can raise CRP/ESR. Additional tests (e.g. ferritin, fibrinogen, albumin, or serum amyloid A) sometimes help characterize the inflammatory state, especially in research settings.
In summary, the modern diagnostic approach to inflammation is multi-faceted: a clinician combines history and physical exam with targeted lab tests and imaging. High ESR/CRP levels, in the right clinical context, confirm inflammation is present; the challenge is determining its source and whether it is acute or chronic. For example, unexplained fever with high CRP and elevated liver enzymes might prompt evaluation for autoimmune hepatitis or chronic infection.
Treatment Strategies
The treatment of inflammation depends on the cause, severity and duration of the process. Broadly, management can be divided into supportive measures, pharmacologic therapy, and lifestyle interventions.
Acute inflammation (supportive care): For localized, short-term inflammation (e.g. a sprained ankle or skin infection), RICE therapy is often sufficient: rest the injured area, apply ice packs, use compression (bandages), and elevate the limb to reduce swelling. Over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen or naproxen can reduce pain and swelling. Antibiotics or surgical drainage may be needed if infection is present. In many mild cases, this simple approach resolves inflammation in a few days.
Medications for chronic inflammation: When inflammation is persistent or systemic, stronger therapies are used. NSAIDs remain first-line for many inflammatory pains (arthritis, tendonitis) because they inhibit cyclooxygenase and reduce prostaglandin-mediated pain and swelling. If NSAIDs are insufficient, corticosteroids (like prednisone or injected methylprednisolone) can suppress immune activity more powerfully. For example, intra-articular steroid injections quickly calm joint flares, while oral steroids treat severe systemic flares (as in lupus or vasculitis). Steroids must be used judiciously due to side effects with long-term use.
Disease-modifying therapies: In immune-mediated diseases, medications that target the underlying process are critical. DMARDs (disease-modifying antirheumatic drugs) such as methotrexate, sulfasalazine, azathioprine and hydroxychloroquine are commonly used in rheumatoid arthritis and other chronic inflammatory conditions. These drugs may take weeks to work but can alter disease course and prevent joint or organ damage. Newer small-molecule agents like JAK inhibitors (e.g. tofacitinib) also block specific immune signaling pathways.
Biologic therapies: A revolution in inflammation treatment has been the advent of biologic drugs – engineered monoclonal antibodies or receptor molecules that block key cytokines or cell interactions. Examples include TNF inhibitors (infliximab, adalimumab, etanercept) used in rheumatoid arthritis, Crohn’s disease and psoriasis, and IL-6 inhibitors (tocilizumab) or IL-1 blockers for conditions like systemic juvenile arthritis. By neutralizing cytokines like TNF-α and IL-6, these agents can dramatically reduce inflammation and symptoms. Biologics are also used in inflammatory bowel disease (e.g. anti-TNF drugs for Crohn’s/UC) and dermatologic conditions (e.g. psoriasis biologics targeting IL-17 or IL-23). A few therapies target immune cells more directly – for instance, rituximab (anti-CD20) depletes B cells in rheumatoid arthritis and vasculitis. These advanced drugs generally require specialist management and monitoring.
Symptomatic and supportive therapies: Pain and dysfunction from inflammation are often treated with analgesics (acetaminophen, NSAIDs) and physical rehabilitation. Physical therapy can help maintain mobility and reduce pain (e.g. gentle exercises for inflamed joints). Local therapies like heating pads or cold packs provide relief for some conditions. In chronic inflammatory diseases, interdisciplinary care (occupational therapy, pain management) is important to preserve quality of life.
Lifestyle and dietary interventions: Because systemic inflammation is strongly influenced by lifestyle, dietary measures are emphasized. An anti-inflammatory diet – rich in fruits, vegetables, whole grains, legumes and omega-3 fatty acids – can modestly lower markers of inflammation. Foods with natural anti-inflammatory compounds (e.g. fatty fish, olive oil, turmeric, ginger) are encouraged. Conversely, patients are advised to avoid trans fats, excessive red meat, refined sugars and processed foods, as these can exacerbate inflammation. Weight loss in overweight individuals often leads to significant decreases in CRP and joint strain. Regular exercise has an anti-inflammatory effect (reducing visceral fat and altering cytokine profiles), so it is routinely recommended for chronic inflammatory patients. Stress reduction techniques (mindfulness, adequate sleep, counselling) may also help, given the link between stress hormones and inflammation. Some clinicians advise dietary supplements – for example, omega-3 fish oil, vitamin D, and certain antioxidants – which have been shown to dampen inflammation in some studies. While supplements should be discussed with a provider, they are a low-risk adjunct to therapy.
Emerging therapies: Research is underway on novel anti-inflammatory treatments. These include targeted small molecules (JAK inhibitors), gene and cell therapies, and even microbiome manipulation. For example, therapies that inhibit the NLRP3 inflammasome or modulate gut bacteria are being explored. At present, most emerging treatments are in specialized use (e.g. JAK inhibitors for rheumatoid arthritis) or clinical trials. However, their development reflects a trend toward precision medicine, where therapy is tailored to the specific inflammatory pathway involved.
Surgery: In certain chronic inflammatory conditions, surgery may be needed. For instance, in severe ulcerative colitis unresponsive to medication, colectomy is curative. Debridement of chronically inflamed tissue (e.g. necrotizing soft tissue infections) is also lifesaving. Joint replacement can address end-stage inflammatory arthritis after years of damage. Thus, although medication is first-line, surgical intervention sometimes plays a role.
Prevention and Public Health Implications
Preventing unnecessary inflammation is an important public health goal, since chronic inflammation underlies many leading causes of morbidity and mortality. Globally, cardiovascular disease, cancer and type 2 diabetes (all linked to inflammation) account for the majority of deaths. Efforts to reduce obesity, smoking and sedentary behavior are therefore also seen as anti-inflammatory public health measures. For example, public campaigns promoting a Mediterranean-type diet (high in plants and healthy oils) and regular exercise can lower population-level inflammation markers.
On an individual level, recommended preventive strategies include:
Healthy lifestyle: Maintaining a healthy weight, exercising regularly, and eating a balanced, fiber-rich diet. These measures lower visceral fat (which secretes inflammatory cytokines) and ensure a balanced gut microbiome.
Avoiding exposure: Reducing exposure to pollutants, occupational irritants, and tobacco smoke prevents the initiation of inflammatory cascades in the lungs and other organs. For instance, air quality regulations can lower the incidence of chronic airway inflammation.
Vaccination and infection control: By preventing infections (through vaccines, hygiene, safe food practices), we reduce episodes of acute inflammation and potential triggers for chronic sequelae. Some chronic inflammatory conditions have infectious precedents (e.g. hepatitis vaccines prevent chronic liver inflammation).
Regular health screening: Monitoring chronic diseases (hypertension, diabetes, obesity) and treating them can indirectly reduce systemic inflammation. For example, controlling blood sugar in diabetes lowers the inflammatory complications of hyperglycemia.
Clinically, early recognition of chronic inflammation (through screenings like CRP in high-risk individuals) and treating underlying causes can prevent progression to severe disease. Governments and health agencies recognize inflammation’s role in chronic disease. Interventions such as sugar taxes, smoking bans, and community exercise programs all aim to reduce baseline inflammation in the population.
Physiological vs. Pathological Inflammation
It is crucial to distinguish physiological (normal) inflammation from pathological (disease-causing) inflammation. Normal inflammation is the body’s healing response: it occurs only when needed and resolves itself. A fever from a benign infection or the redness around a healing wound are physiologic – evidence that the immune system is working. In contrast, pathological inflammation is dysregulated, occurring inappropriately or excessively. For example, chronic inflammation in an otherwise healthy tissue (such as the arterial wall in atherosclerosis) is pathological, because it serves no protective purpose and instead causes harm. In autoimmunity, inflammation targets normal cells – also a pathological process. Statistically, chronic inflammatory diseases (atherosclerosis, diabetes, Alzheimer’s, etc.) are responsible for over half of deaths worldwide, underscoring the need to control inflammation medically and behaviorally.
Healthcare professionals must therefore treat inflammation contextually. Suppressing inflammation is desirable when it threatens health (autoimmune flares, cytokine storms), but unnecessary immunosuppression can be harmful. The goal is to restore balance – as one textbook notes, the ultimate goal of inflammation is to restore homeostasis. When that balance tips toward chronic, self-perpetuating inflammation, targeted interventions are required to prevent tissue damage and complications.